
Chemistry, 11.03.2020 23:36 kandrews6221
A sample of nitrogen gas had a volume of 500. mL, a pressure in its closed container of 740 torr, and a temperature of 25 °C. What was the new volume of the gas when the temperature was changed to 50 °C and the new pressure is 760 torr

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
A sample of nitrogen gas had a volume of 500. mL, a pressure in its closed container of 740 torr, an...
Questions


Physics, 04.10.2021 22:10

Business, 04.10.2021 22:10


SAT, 04.10.2021 22:10



Biology, 04.10.2021 22:10




SAT, 04.10.2021 22:10



Spanish, 04.10.2021 22:10



Mathematics, 04.10.2021 22:10



 = initial pressure of gas = 740 torr
= initial pressure of gas = 740 torr = final pressure of gas = 760 torr
= final pressure of gas = 760 torr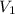 = initial volume of gas = 500 mL
= initial volume of gas = 500 mL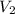 = final volume of gas = ?
= final volume of gas = ? = initial temperature of gas =
= initial temperature of gas = 
 = final temperature of gas =
= final temperature of gas = 




