
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . Suppose 67.7 g of sulfuric acid is mixed with 83. g of sodium hydroxide. Calculate the minimum mass of sulfuric acid that could be left over by the chemical reaction. Round your answer to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 23.06.2019 09:30
Organisms that live in the alpine and taiga biomes have developed unique adaptations that aid in their survival. moss campion is one of the plants found in the alpine biome. it has small leaves and a cushion shape that protect it from the wind and freezing temperatures in the alpine. how has the moss campion adapted to enable its survival in the alpine biome? a. waxy needles b. cone-shaped c. thin trunks d. low-growing
Answers: 1
You know the right answer?
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and l...
Questions

English, 22.02.2021 21:50




Spanish, 22.02.2021 21:50

History, 22.02.2021 21:50

Mathematics, 22.02.2021 21:50

Mathematics, 22.02.2021 21:50


Physics, 22.02.2021 21:50


Mathematics, 22.02.2021 21:50

English, 22.02.2021 21:50

Social Studies, 22.02.2021 21:50


History, 22.02.2021 21:50

Mathematics, 22.02.2021 21:50

History, 22.02.2021 21:50

Mathematics, 22.02.2021 21:50

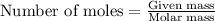
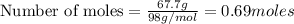
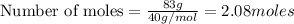
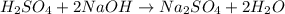
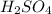 require 2 moles of
require 2 moles of 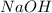
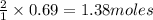 of
of 

