The law of conservation of mass states that in a chemical reaction the mass of the reactants
eq...

Chemistry, 12.03.2020 00:31 GxthGrl6612
The law of conservation of mass states that in a chemical reaction the mass of the reactants
equals the mass of the products. Based on this information, what mass of hydrogen (H2)
was produced in this reaction?
A. 2 g
B. 4 g
C. 72 g
D. 144 g
please leave an explanation
thank you!
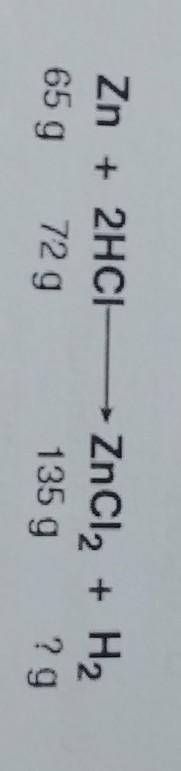

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
Questions

Mathematics, 19.11.2020 07:40

Chemistry, 19.11.2020 07:40

Mathematics, 19.11.2020 07:40

Computers and Technology, 19.11.2020 07:40



Health, 19.11.2020 07:40



Computers and Technology, 19.11.2020 07:40


Mathematics, 19.11.2020 07:40


Mathematics, 19.11.2020 07:40

Mathematics, 19.11.2020 07:40


Health, 19.11.2020 07:40

English, 19.11.2020 07:40


Spanish, 19.11.2020 07:40



