
Chemistry, 12.03.2020 01:53 adrian08022
A buffered solution containing dissolved aniline, C 6 H 5 NH 2 , and aniline hydrochloride, C 6 H 5 NH 3 Cl , has a pH of 5.34 . A. Determine the concentration of C 6 H 5 NH 3 in the solution if the concentration of C 6 H 5 NH 2 is 0.245 M. The p K b of aniline is 9.13.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3


Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
A buffered solution containing dissolved aniline, C 6 H 5 NH 2 , and aniline hydrochloride, C 6 H 5...
Questions


Mathematics, 17.10.2019 01:30

History, 17.10.2019 01:30


Mathematics, 17.10.2019 01:30

Mathematics, 17.10.2019 01:30



History, 17.10.2019 01:30

Computers and Technology, 17.10.2019 01:30


English, 17.10.2019 01:30


Health, 17.10.2019 01:30

Mathematics, 17.10.2019 01:30

History, 17.10.2019 01:30

History, 17.10.2019 01:30


Biology, 17.10.2019 01:30

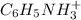 is, 0.0830 M
is, 0.0830 M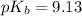
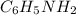 = 0.245 M
= 0.245 M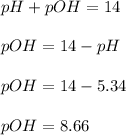
![pOH=pK_b+\log \frac{[Salt]}{[Base]}](/tpl/images/0544/0242/ac570.png)
![pOH=pK_b+\log \frac{[C_6H_5NH_3^+]}{[C_6H_5NH_2]}](/tpl/images/0544/0242/af8e2.png)
![8.66=9.13+\log (\frac{[C_6H_5NH_3^+]}{0.245})](/tpl/images/0544/0242/99488.png)
![[C_6H_5NH_3^+]=0.0830M](/tpl/images/0544/0242/f1802.png)


