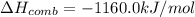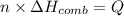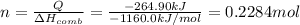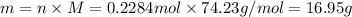Chemistry, 12.03.2020 01:59 cordovatierra16
If the heat of combustion for a specific compound is − 1160.0 kJ / mol and its molar mass is 74.23 g / mol, how many grams of this compound must you burn to release 264.90 kJ of heat?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 00:40
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
If the heat of combustion for a specific compound is − 1160.0 kJ / mol and its molar mass is 74.23 g...
Questions

English, 09.11.2020 20:20

English, 09.11.2020 20:20

Chemistry, 09.11.2020 20:20




Chemistry, 09.11.2020 20:20

Biology, 09.11.2020 20:20




Mathematics, 09.11.2020 20:20

Mathematics, 09.11.2020 20:20

English, 09.11.2020 20:20

Mathematics, 09.11.2020 20:20


Mathematics, 09.11.2020 20:20

Geography, 09.11.2020 20:20

Biology, 09.11.2020 20:20

Social Studies, 09.11.2020 20:20