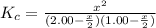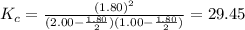Chemistry, 12.03.2020 02:05 martinbricein10
2.00 mol of H2(g) and 1.00 mol of I2(g) are placed in a 1.00 L container, and they react to form HI(g). At equilibrium, it is found that 1.80 moles of HI(g) are present in the container. Calculate K for the reaction: H2(g) + I2(g) ⇄ 2 HI(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which of the following mining methods disrupts the sea floor?
Answers: 1

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
2.00 mol of H2(g) and 1.00 mol of I2(g) are placed in a 1.00 L container, and they react to form HI(...
Questions







Mathematics, 30.10.2021 14:00


Computers and Technology, 30.10.2021 14:00

Computers and Technology, 30.10.2021 14:00

Biology, 30.10.2021 14:00



Mathematics, 30.10.2021 14:00

Mathematics, 30.10.2021 14:00



History, 30.10.2021 14:00

Business, 30.10.2021 14:00

Business, 30.10.2021 14:00

![[H_2]= \frac{2.00 mol}{1.00 L}=2.00 M](/tpl/images/0544/0690/a78aa.png)
![[I_2]= \frac{I.00 mol}{1.00 L}=1.00 M](/tpl/images/0544/0690/94c40.png)
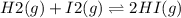
![[HI]=\frac{1.80 mol}{1.00L} = 1.80M= x](/tpl/images/0544/0690/bcad7.png)
![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0544/0690/62646.png)