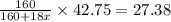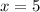Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3



Chemistry, 23.06.2019 16:00
Afuel has 30.43% nitrogen and 69.57% oxygen. find the molecular formula of the compound if it has a mass of 92 grams per mole a.no b.n2o4 c.no2 d.n4o8
Answers: 1
You know the right answer?
A chemist is given a sample of the CuSO4 hydrate and asked to determine its the empirical formula. T...
Questions

Mathematics, 12.06.2020 07:57

Mathematics, 12.06.2020 07:57




Mathematics, 12.06.2020 07:57

Mathematics, 12.06.2020 07:57

Mathematics, 12.06.2020 07:57

Mathematics, 12.06.2020 07:57




Mathematics, 12.06.2020 07:57

Mathematics, 12.06.2020 07:57



Biology, 12.06.2020 07:57

History, 12.06.2020 07:57

History, 12.06.2020 07:57

Mathematics, 12.06.2020 07:57

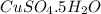
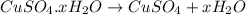
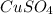 = 160 g/mol
= 160 g/mol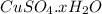 decomposes to give 160 g of anhydrous
decomposes to give 160 g of anhydrous 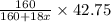 g of anhydrous
g of anhydrous