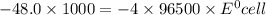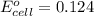Chemistry, 12.03.2020 02:06 stranger123
Calculate the standard potential, E ∘ , E°, for this reaction from its Δ G ∘ ΔG° value. X ( s ) + Y 4 + ( aq ) ⟶ X 4 + ( aq ) + Y ( s ) Δ G ∘ = − 48.0 kJ

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3


Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Calculate the standard potential, E ∘ , E°, for this reaction from its Δ G ∘ ΔG° value. X ( s ) + Y...
Questions


Mathematics, 26.09.2021 03:00


Mathematics, 26.09.2021 03:00


History, 26.09.2021 03:00


Law, 26.09.2021 03:00

Mathematics, 26.09.2021 03:00

Biology, 26.09.2021 03:00

Mathematics, 26.09.2021 03:00


Mathematics, 26.09.2021 03:00


Chemistry, 26.09.2021 03:00


Chemistry, 26.09.2021 03:00

Mathematics, 26.09.2021 03:00


History, 26.09.2021 03:00

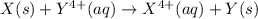
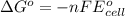
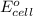 = standard cell potential = ?
= standard cell potential = ?