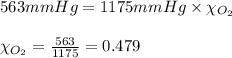Chemistry, 12.03.2020 02:35 meganwintergirl
The partial pressures of CH4, N2, and O2 in a sample of gas were found to be 143 mmHg, 469 mmHg, and 563 mmHg, respectively. Calculate the mole fraction of oxygen. 20.1 0.399 0.741 0.479 0.359

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
The partial pressures of CH4, N2, and O2 in a sample of gas were found to be 143 mmHg, 469 mmHg, and...
Questions

Physics, 10.03.2020 06:14






Social Studies, 10.03.2020 06:14


Biology, 10.03.2020 06:14

Mathematics, 10.03.2020 06:14










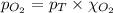
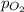 = partial pressure of oxygen gas = 563 mmHg
= partial pressure of oxygen gas = 563 mmHg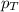 = total pressure = 1175 mmHg
= total pressure = 1175 mmHg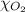 = mole fraction of oxygen gas = ?
= mole fraction of oxygen gas = ?