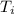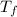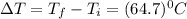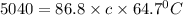Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which statement accurately describes the rock layers? layer 8 is older than layer 1. layer 3 is younger than layer 6. layer 4 and layer 10 are the same relative age. layer 2 and layer 9 are the same relative age.
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3


You know the right answer?
An unknown metal has a mass of 86.8 g. When 5040 J of heat are added to the sample, the sample tempe...
Questions

Mathematics, 01.12.2020 19:00

English, 01.12.2020 19:00

Computers and Technology, 01.12.2020 19:00

Geography, 01.12.2020 19:00

Social Studies, 01.12.2020 19:00


Mathematics, 01.12.2020 19:00







Social Studies, 01.12.2020 19:00




Mathematics, 01.12.2020 19:00


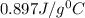
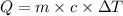
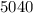 Joules
Joules