

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
You have 32.8 g of O2 gas in a container with twice the volume as one with CO2 gas. The pressure and...
Questions



Computers and Technology, 07.04.2020 20:23

English, 07.04.2020 20:23

English, 07.04.2020 20:23


Mathematics, 07.04.2020 20:23


Health, 07.04.2020 20:23





Biology, 07.04.2020 20:23

Mathematics, 07.04.2020 20:23

English, 07.04.2020 20:23



Mathematics, 07.04.2020 20:23

Mathematics, 07.04.2020 20:23

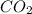 gas in container = 22.56 g
gas in container = 22.56 g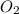 = 32 g/mol
= 32 g/mol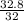 moles of
moles of 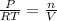
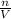 ratio for both
ratio for both 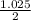 moles = 0.5125 moles
moles = 0.5125 moles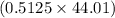 g = 22.56 g
g = 22.56 g

