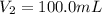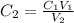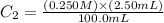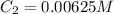Chemistry, 12.03.2020 03:01 toledanomariap43bxm
Determine the concentration of the following dye standard made by a student who pipettes out 2.50 mL of a 0.250 M stock solution and transfers it into a volumetric flask and dilutes the dye to a final volume of 100.0 mL with DI water.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Determine the concentration of the following dye standard made by a student who pipettes out 2.50 mL...
Questions







Mathematics, 29.08.2020 01:01



Mathematics, 29.08.2020 01:01

Mathematics, 29.08.2020 01:01

Spanish, 29.08.2020 01:01

Spanish, 29.08.2020 01:01

Mathematics, 29.08.2020 01:01


History, 29.08.2020 01:01

Health, 29.08.2020 01:01


Mathematics, 29.08.2020 01:01

Mathematics, 29.08.2020 01:01

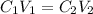
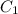 and
and 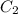 are initial and final concentration respectively
are initial and final concentration respectively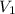 and
and 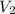 are initial and final volume respectively
are initial and final volume respectively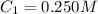 ,
, 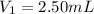 and
and