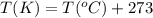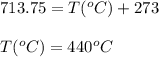Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1
You know the right answer?
A sample of gas has a volume of 1.24 L under 2.35 atm pressure at 45°C. If the gas is then expanded...
Questions

Geography, 19.09.2019 20:00

Geography, 19.09.2019 20:00


Mathematics, 19.09.2019 20:00




English, 19.09.2019 20:00

Computers and Technology, 19.09.2019 20:00








Physics, 19.09.2019 20:00

Advanced Placement (AP), 19.09.2019 20:00


English, 19.09.2019 20:00

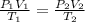
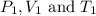 are the initial pressure, volume and temperature of the gas
are the initial pressure, volume and temperature of the gas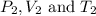 are the final pressure, volume and temperature of the gasW
are the final pressure, volume and temperature of the gasW![P_1=2.35atm\\V_1=1.24L\\T_1=45^oC=[45+273]K=318K\\P_2=0.515atm\\V_2=12.7L\\T_2=?](/tpl/images/0544/2731/0dccc.png)