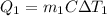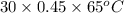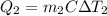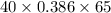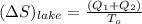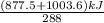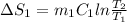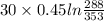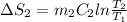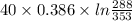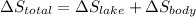Chemistry, 12.03.2020 05:14 Jwhite8602
A 30-kg iron block and a 40-kg copper block, both initially at 808C, are dropped into a large lake at 158C. Thermal equilibrium is established after a while as a result of heat transfer between the blocks and the lake water. Determine the total entropy change for this process.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
A 30-kg iron block and a 40-kg copper block, both initially at 808C, are dropped into a large lake a...
Questions

Social Studies, 08.09.2020 04:01

Mathematics, 08.09.2020 04:01



Mathematics, 08.09.2020 04:01

Mathematics, 08.09.2020 04:01

Spanish, 08.09.2020 04:01

Mathematics, 08.09.2020 04:01

English, 08.09.2020 04:01


Mathematics, 08.09.2020 04:01

Mathematics, 08.09.2020 04:01

History, 08.09.2020 04:01

Mathematics, 08.09.2020 04:01


Mathematics, 08.09.2020 04:01

Mathematics, 08.09.2020 04:01



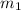 = 30 kg,
= 30 kg, 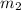 = 40 kg
= 40 kg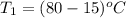 =
= 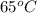
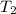 =
=