

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Predict the direction in which the equilibrium will lie for the reaction H2SO3(aq) + HCO3- (aq) <...
Questions

Social Studies, 29.01.2022 01:00


Mathematics, 29.01.2022 01:00


Mathematics, 29.01.2022 01:00

Mathematics, 29.01.2022 01:00


Mathematics, 29.01.2022 01:00




Mathematics, 29.01.2022 01:00


Mathematics, 29.01.2022 01:00



Mathematics, 29.01.2022 01:00



Mathematics, 29.01.2022 01:00

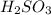 is a strong acid as compared to carbonic acid. As a result, sulfurous acid (
is a strong acid as compared to carbonic acid. As a result, sulfurous acid (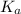 of
of 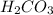 .
.

