
At −11.0 ∘C , a common temperature for household freezers, what is the maximum mass of sucrose (C12H22O11) you can add to 3.00 kg of pure water and still have the solution freeze? Assume that sucrose is a molecular solid and does not ionize when it dissolves in water.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
At −11.0 ∘C , a common temperature for household freezers, what is the maximum mass of sucrose (C12H...
Questions



Health, 25.09.2020 23:01

Chemistry, 25.09.2020 23:01


Physics, 25.09.2020 23:01

Biology, 25.09.2020 23:01

Arts, 25.09.2020 23:01

Mathematics, 25.09.2020 23:01

Physics, 25.09.2020 23:01

Mathematics, 25.09.2020 23:01

Social Studies, 25.09.2020 23:01

History, 25.09.2020 23:01

History, 25.09.2020 23:01





Mathematics, 25.09.2020 23:01

Chemistry, 25.09.2020 23:01

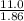 = 5.91 m.
= 5.91 m.
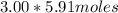 (= 17.73 moles) to the water to get the -11.0° C freezing point depression.
(= 17.73 moles) to the water to get the -11.0° C freezing point depression.


