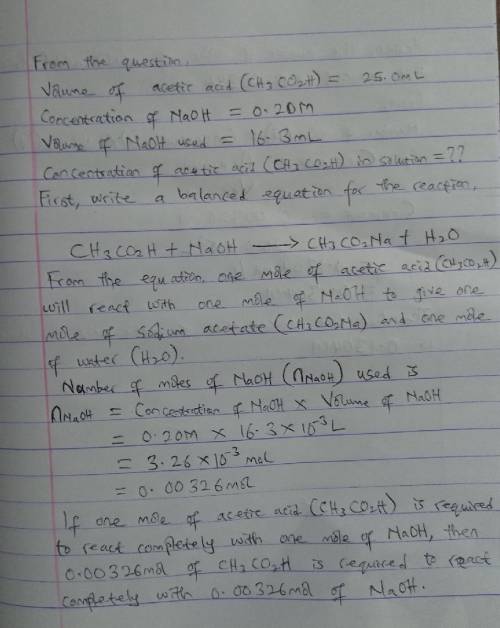Chemistry, 12.03.2020 06:08 JavyHart9695
A 25.0mL solution acetic acid (CH3CO2H) is titrated with 0.20M NaOH and reaches the endpoint after the addition of 16.3mL of NaOH. What is the concentration of acetic acid in solution

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
A 25.0mL solution acetic acid (CH3CO2H) is titrated with 0.20M NaOH and reaches the endpoint after t...
Questions

Health, 20.10.2020 23:01



English, 20.10.2020 23:01




Mathematics, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01

History, 20.10.2020 23:01



Social Studies, 20.10.2020 23:01

Social Studies, 20.10.2020 23:01





Health, 20.10.2020 23:01

Mathematics, 20.10.2020 23:01