ASAP!!
Determine whether or not the equation below is balanced. If it isn’t balanced, write t...

ASAP!!
Determine whether or not the equation below is balanced. If it isn’t balanced, write the balanced form. Also, identify the reactant(s) and product(s) in this equation. Finally, label this as one of the five types of reactions: combination, decomposition, substitution, double replacement, or reversible.
Zn+HCl→ZnCl2+H2
2. Determine whether or not the equation below is balanced. If it isn’t balanced, write the balanced form. Also, identify the reactant(s) and product(s) in this equation. Finally, label this as one of the five types of reactions: combination, decomposition, substitution, double replacement, or reversible.
S8+24F2→8SF6
3. Calculate the molecular mass of ferric oxide (Fe3O4).
4. Determine the percentage composition of chlorine in CaCl2.
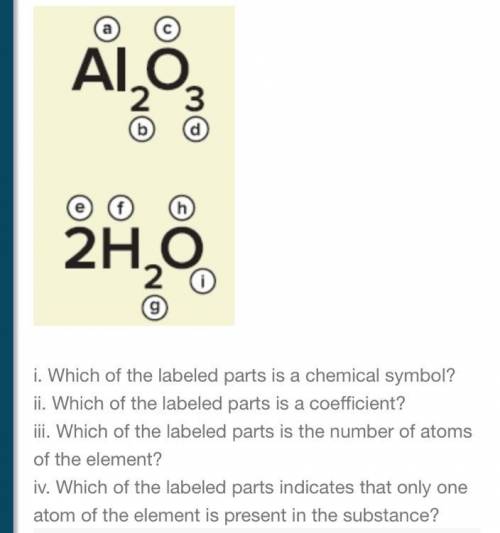
5. Identify the labeled parts in the figure.

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1


You know the right answer?
Questions

Physics, 07.10.2019 09:10


English, 07.10.2019 09:10



Physics, 07.10.2019 09:10

Chemistry, 07.10.2019 09:10




History, 07.10.2019 09:10





Mathematics, 07.10.2019 09:10

German, 07.10.2019 09:10

History, 07.10.2019 09:10


Physics, 07.10.2019 09:10



