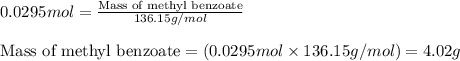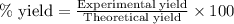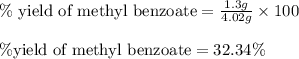Chemistry, 12.03.2020 16:55 sfigel3160
A reaction was performed in which 3.6 g 3.6 g of benzoic acid was reacted with excess methanol to make 1.3 g 1.3 g of methyl benzoate. Calculate the theoretical yield and percent yield for this reaction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2
You know the right answer?
A reaction was performed in which 3.6 g 3.6 g of benzoic acid was reacted with excess methanol to ma...
Questions

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Social Studies, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

English, 14.09.2020 01:01

English, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Physics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

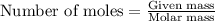 .....(1)
.....(1)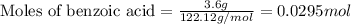
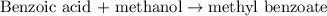
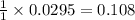 moles of methyl benzoate
moles of methyl benzoate