Chemistry, 12.03.2020 17:25 aubreymoore4553
What is the value of the van't Hoff factor for KCl if a 1.00m aqueous solution shows a vapor pressure depression of 0.734 mmHg at 298 ∘C? (The vapor pressure of water at 298 K is 23.76 mmHg.)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

You know the right answer?
What is the value of the van't Hoff factor for KCl if a 1.00m aqueous solution shows a vapor pressur...
Questions






Computers and Technology, 22.07.2019 20:30

History, 22.07.2019 20:30





Mathematics, 22.07.2019 20:30

Geography, 22.07.2019 20:30





Mathematics, 22.07.2019 20:30


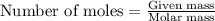
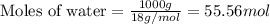
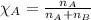
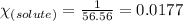
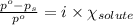
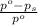 = relative lowering in vapor pressure = 0.734 mmHg
= relative lowering in vapor pressure = 0.734 mmHg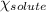 = mole fraction of solute = 0.0177
= mole fraction of solute = 0.0177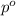 = vapor pressure of pure water = 23.76 torr
= vapor pressure of pure water = 23.76 torr