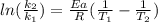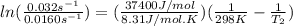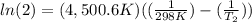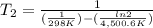Chemistry, 12.03.2020 20:03 shelbylynn17
The activation energy of a certain reaction is 37.4 kJ/mol . At 25 ∘C , the rate constant is 0.0160s−1 . At what temperature in degrees Celsius would this reaction go twice as fast?

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 09:00
Need ! assume that the variables x and y are directly related. if k = 8, what is the value for each of the following points? be sure and record your data to be used in the following problem. x y k 0.
Answers: 2


Chemistry, 23.06.2019 18:30
Calcium hydroxide and hydrochloric acid react to form calcium chloride and water as shown in the chemical reaction. if the chemicals are present in exactly the correct ratios to fully use all of the ingredients, how many moles of water would be formed from 5 moles of hcl? (oh)2 + ? +
Answers: 1

Chemistry, 24.06.2019 01:30
C8h18(g) + o2(g) →co2(g)+h2o(g) how many grams of oxygen are required to react with 17.0 grams of octane (c8h18) in the combustion of octane in gasoline?
Answers: 3
You know the right answer?
The activation energy of a certain reaction is 37.4 kJ/mol . At 25 ∘C , the rate constant is 0.0160s...
Questions


Mathematics, 02.02.2021 05:10



History, 02.02.2021 05:10




Mathematics, 02.02.2021 05:10

Mathematics, 02.02.2021 05:10


History, 02.02.2021 05:10

Chemistry, 02.02.2021 05:10

Advanced Placement (AP), 02.02.2021 05:10




Mathematics, 02.02.2021 05:10

Mathematics, 02.02.2021 05:10

Mathematics, 02.02.2021 05:10