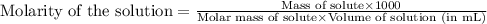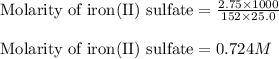Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
An aqueous solution of iron(II) sulfate (FeSO4) is prepared by dissolving 2.75 g in sufficient deion...
Questions

Biology, 03.04.2021 01:00

History, 03.04.2021 01:00

Mathematics, 03.04.2021 01:00

History, 03.04.2021 01:00




Mathematics, 03.04.2021 01:00

Mathematics, 03.04.2021 01:00



Mathematics, 03.04.2021 01:00

Health, 03.04.2021 01:00

Physics, 03.04.2021 01:00





Mathematics, 03.04.2021 01:00