
Chemistry, 12.03.2020 20:19 anonymous9723
At a certain temperature, the equilibrium constant, K c , for this reaction is 53.3. H 2 ( g ) + I 2 ( g ) − ⇀ ↽ − 2 HI ( g ) K c = 53.3 At this temperature, 0.600 mol H 2 and 0.600 mol I 2 were placed in a 1.00 L container to react. What concentration of HI is present at equilibrium?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1



Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
At a certain temperature, the equilibrium constant, K c , for this reaction is 53.3. H 2 ( g ) + I 2...
Questions



English, 05.10.2020 20:01

Mathematics, 05.10.2020 20:01

Social Studies, 05.10.2020 20:01





Medicine, 05.10.2020 20:01



Spanish, 05.10.2020 20:01


Mathematics, 05.10.2020 20:01

Mathematics, 05.10.2020 20:01



Mathematics, 05.10.2020 20:01

![[HI]_{eq}=0.942M](/tpl/images/0545/3123/a6e19.png)
![[H_2]_0=[I_2]_0=0.600M](/tpl/images/0545/3123/01527.png)
 due to the chemical change as shown below:
due to the chemical change as shown below: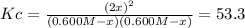
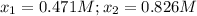
![[HI]_{eq}=2*0.471M=0.942M](/tpl/images/0545/3123/1e73e.png)



