
Chemistry, 12.03.2020 20:27 eduardoma2902
At a certain temperature the value of the equilibrium constant, Kc, is 1.27 for the reaction. 2As (s) + 3H2 (g) ⇌ 2AsH3 (g) What is the value of Kc for the following reaction? AsH3 (g) ⇌ As (s) + 3/2H2 (g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
At a certain temperature the value of the equilibrium constant, Kc, is 1.27 for the reaction. 2As (s...
Questions

Advanced Placement (AP), 07.05.2021 21:00




English, 07.05.2021 21:00



History, 07.05.2021 21:00

Mathematics, 07.05.2021 21:00


Chemistry, 07.05.2021 21:00

History, 07.05.2021 21:00

Mathematics, 07.05.2021 21:00




Mathematics, 07.05.2021 21:00


Mathematics, 07.05.2021 21:00

History, 07.05.2021 21:00

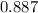
![Kc=\frac{[AsH_3]^2}{[As]^2[H_2] ^3}=1.27](/tpl/images/0545/3503/09ec8.png)
![Kc=\frac{[As][H_2] ^{3/2}}{[AsH_3]}](/tpl/images/0545/3503/b2ae8.png)
![\sqrt{Kc} =\sqrt{\frac{[AsH_3]^2}{[As]^2[H_2] ^3}} =\sqrt{1.27}](/tpl/images/0545/3503/dc32b.png)
![\frac{\sqrt{[AsH_3]^2} }{\sqrt{[As]^2} \sqrt{[H_2] ^3} } =\sqrt{1.27}](/tpl/images/0545/3503/19f81.png)
![\sqrt{1.27}=\frac{[AsH_3]}{[As][H_2] ^{3/2}}](/tpl/images/0545/3503/e4fe4.png)
![Kc'=\frac{1}{\sqrt{1.27} }=\frac{[As][H_2] ^{3/2}}{[AsH_3]}=0.887](/tpl/images/0545/3503/a88a2.png)


