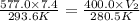A certain flexible weather balloon contains 7.4 L of helium gas. Initially, the balloon is in WP at 8500ft, where the temperature is 20.6oC and the barometric pressure is 577.0 torr. The balloon then is taken to the top of Pike’s Peak at an altitude of 14,100ft, where the pressure is 400 torr and the temperature is 7.5oC. What is the new volume of the balloon at the top of Pikes Peak?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

You know the right answer?
A certain flexible weather balloon contains 7.4 L of helium gas. Initially, the balloon is in WP at...
Questions







History, 30.11.2021 14:00

Mathematics, 30.11.2021 14:00

Biology, 30.11.2021 14:00






Social Studies, 30.11.2021 14:00



English, 30.11.2021 14:00



 = initial pressure of gas = 577.0 torr
= initial pressure of gas = 577.0 torr = final pressure of gas = 400 torr
= final pressure of gas = 400 torr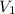 = initial volume of gas = 7.4 L
= initial volume of gas = 7.4 L = final volume of gas = ?
= final volume of gas = ?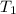 = initial temperature of gas =
= initial temperature of gas = 
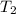 = final temperature of gas =
= final temperature of gas =