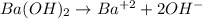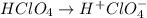Chemistry, 13.03.2020 02:26 valeriegarcia12
An aqueous solution of barium hydroxide is standardized by titration with a 0.167 M solution of perchloric acid. If 12.8 mL of base are required to neutralize 25.4 mL of the acid, what is the molarity of the barium hydroxide solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
An aqueous solution of barium hydroxide is standardized by titration with a 0.167 M solution of perc...
Questions

Mathematics, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01


Computers and Technology, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01






History, 01.07.2020 15:01




Physics, 01.07.2020 15:01