Chemistry, 13.03.2020 04:53 maevemboucher78
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.650 M, [B] = 1.35 M, and [C] = 0.300 M. The following reaction occurs and equilibrium is established: A+2B<->C
At equilibrium, [A] = 0.550 M and [B] = 0.400 M. Calculate the value of the equilibrium constant, Kc

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

You know the right answer?
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.650 M, [B] = 1.35...
Questions



Mathematics, 28.03.2020 07:17



Mathematics, 28.03.2020 07:17

Mathematics, 28.03.2020 07:17

Geography, 28.03.2020 07:17

Mathematics, 28.03.2020 07:18

Mathematics, 28.03.2020 07:18

Biology, 28.03.2020 07:18

Mathematics, 28.03.2020 07:18





Mathematics, 28.03.2020 07:20

Mathematics, 28.03.2020 07:20

Mathematics, 28.03.2020 07:20

History, 28.03.2020 07:21

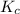 for given reaction is 0.465
for given reaction is 0.465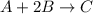
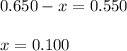
![K_c=\frac{[C]}{[A][B]^2}](/tpl/images/0546/2117/240ef.png)