
Chemistry, 13.03.2020 04:31 Penelope9687
An iron bar weighed 565 g. After the bar had been standing in moist air for a month, exactly one-eighth of the iron turned to rust (Fe2O3). Calculate the final mass of the iron bar and rust.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
An iron bar weighed 565 g. After the bar had been standing in moist air for a month, exactly one-eig...
Questions

Advanced Placement (AP), 31.08.2021 19:50



World Languages, 31.08.2021 19:50

Physics, 31.08.2021 19:50

Physics, 31.08.2021 19:50


Mathematics, 31.08.2021 19:50

Engineering, 31.08.2021 19:50




English, 31.08.2021 19:50

Business, 31.08.2021 19:50

Mathematics, 31.08.2021 19:50

History, 31.08.2021 19:50


Physics, 31.08.2021 19:50

Mathematics, 31.08.2021 19:50

Spanish, 31.08.2021 19:50

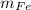 ) = 565 ×
) = 565 × 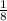 = 70.625 g
= 70.625 g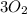 →
→ 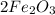
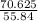 = 1.26 mol
= 1.26 mol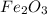 = 159.68 g
= 159.68 g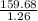 = 126.730 g
= 126.730 g 

