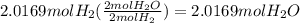27. For the reaction 2 H2 + O2 → 2 H20, how
many moles of H2O would you get from 2.0169
...


Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
Questions


Social Studies, 21.07.2019 22:30

Health, 21.07.2019 22:30

Social Studies, 21.07.2019 22:30




History, 21.07.2019 22:30

Mathematics, 21.07.2019 22:30


Advanced Placement (AP), 21.07.2019 22:30


Mathematics, 21.07.2019 22:30

History, 21.07.2019 22:30

History, 21.07.2019 22:30

Geography, 21.07.2019 22:30


SAT, 21.07.2019 22:30

Biology, 21.07.2019 22:30

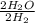 . The twos reduce, making this a one-to-one ratio.
. The twos reduce, making this a one-to-one ratio.