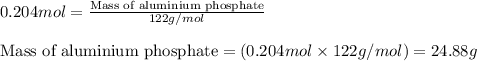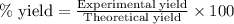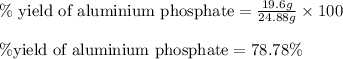Chemistry, 13.03.2020 05:04 cheerleaderautumnche
What is the percent yield of aluminum phosphate if a solution containing 33.4 g of sodium phosphate produced 19.6 g of aluminum phosphate when reacted with excess aluminum chloride in solution

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
What is the percent yield of aluminum phosphate if a solution containing 33.4 g of sodium phosphate...
Questions

Biology, 22.06.2020 23:57



History, 22.06.2020 23:57


Mathematics, 22.06.2020 23:57


Mathematics, 22.06.2020 23:57



Mathematics, 22.06.2020 23:57

Health, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57


Mathematics, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57


History, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57

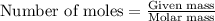 .....(1)
.....(1)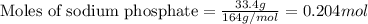
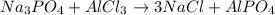
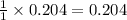 moles of aluminium phosphate
moles of aluminium phosphate