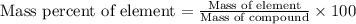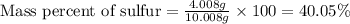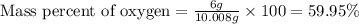Chemistry, 13.03.2020 17:28 anishivaturi123
When 4.008 g of sulfur is burned completely in abundant air, it combines with oxygen to form a single gaseous product with a total mass of 10.008 g. The mass percent of sulfur in the product is % (2 dec places). The mass percent of oxygen in the product is % (2 dec places).

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1
You know the right answer?
When 4.008 g of sulfur is burned completely in abundant air, it combines with oxygen to form a singl...
Questions





Geography, 15.12.2020 19:50

Biology, 15.12.2020 19:50

History, 15.12.2020 19:50

Biology, 15.12.2020 19:50


Mathematics, 15.12.2020 19:50


English, 15.12.2020 19:50

Mathematics, 15.12.2020 19:50


English, 15.12.2020 19:50


Mathematics, 15.12.2020 19:50

Mathematics, 15.12.2020 19:50


Mathematics, 15.12.2020 19:50