
Chemistry, 13.03.2020 18:07 elliswilliams6035
Consider the following reaction: Consider the reaction 2NO(g)+Br2(g)⇌2NOBr(g) ,Kp=28.4 at 298 K In a reaction mixture at equilibrium, the partial pressure of NO is 119 torr and that of Br2 is 151 torr . What is the partial pressure of NOBr in this mixture?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
You know the right answer?
Consider the following reaction: Consider the reaction 2NO(g)+Br2(g)⇌2NOBr(g) ,Kp=28.4 at 298 K In a...
Questions

Mathematics, 19.08.2021 05:50



Mathematics, 19.08.2021 05:50

Computers and Technology, 19.08.2021 05:50

Mathematics, 19.08.2021 05:50




Mathematics, 19.08.2021 06:00

Social Studies, 19.08.2021 06:00

Physics, 19.08.2021 06:00



Social Studies, 19.08.2021 06:00




Chemistry, 19.08.2021 06:00

Mathematics, 19.08.2021 06:00

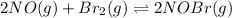
![K_{p}=\frac{[p_{NOBr}]^2}{[p_{NO}]^2\times [p_{Br_2}]^1}](/tpl/images/0546/6296/30bec.png)
![28.4=\frac{[p_{NOBr}]^2}{[(119)^2\times (151)^1}](/tpl/images/0546/6296/6ad3d.png)
![[p_{NOBr}]=7792](/tpl/images/0546/6296/2a41f.png) atm
atm

