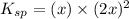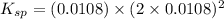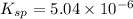Chemistry, 13.03.2020 18:35 ilovecatsomuchlolol
Excess Ca(OH)2 is shaken with water to produce a saturated solution. The solution is filtered, and a 50.00 mL sample titrated with HCl requires 11.15 mL of 0.0973 M HCl to reach the end point. calculate ksp for Ca(OH)2?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1

Chemistry, 23.06.2019 11:00
Afraction can be converted to a decimal by dividing the denominator into the numerator. use a calculator. divide to convert the fractions from part a to decimals. then describe the pattern you see in the decimal.
Answers: 3
You know the right answer?
Excess Ca(OH)2 is shaken with water to produce a saturated solution. The solution is filtered, and a...
Questions


Mathematics, 24.04.2020 08:02



English, 24.04.2020 08:02

Social Studies, 24.04.2020 08:02

Mathematics, 24.04.2020 08:02



Social Studies, 24.04.2020 08:03

Mathematics, 24.04.2020 08:03


Mathematics, 24.04.2020 08:03

Mathematics, 24.04.2020 08:03

English, 24.04.2020 08:03

Mathematics, 24.04.2020 08:03


Physics, 24.04.2020 08:03


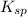 is
is 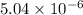
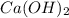 , we use the equation given by neutralization reaction:
, we use the equation given by neutralization reaction: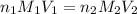
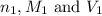 are the n-factor, molarity and volume of
are the n-factor, molarity and volume of 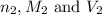 are the n-factor, molarity and volume of HCl.
are the n-factor, molarity and volume of HCl.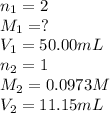
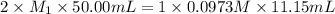
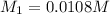

![K_{sp}=[Ca^{2+}][OH^{-}]^2](/tpl/images/0546/6867/4ff7a.png)