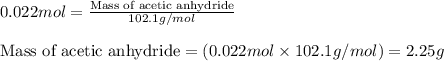Chemistry, 13.03.2020 18:34 chloeann5397
Aspirin is prepared by reaction of salicylic acid (C7H6O3) with acetic anhydride (C4H6O3)according to the following equation:
C7H6O3Salicylicacid+C4H6O3Aceticanh ydride→C9H8O4Aspirin+CH3COOHAcetica cid
a. How many grams of acetic anhydride are needed to react with 3.07 g of salicylic acid?
b. How many grams of aspirin will result?
c. How many grams of acetic acid are formed as a by-product?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Aspirin is prepared by reaction of salicylic acid (C7H6O3) with acetic anhydride (C4H6O3)according t...
Questions


World Languages, 29.01.2020 08:52

Spanish, 29.01.2020 08:52

Chemistry, 29.01.2020 08:52

Mathematics, 29.01.2020 08:52

Physics, 29.01.2020 08:52

Mathematics, 29.01.2020 08:52



History, 29.01.2020 08:53







Mathematics, 29.01.2020 08:53



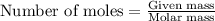 .....(1)
.....(1)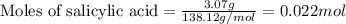
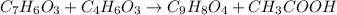
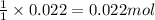 of acetic anhydride
of acetic anhydride