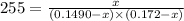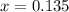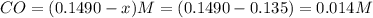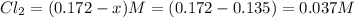CO (g) + Cl2 (g) ⇌ COCl2 (g)

Chemistry, 13.03.2020 19:17 owenbarrows
For the following reaction, Kc = 255 at 1000 K.
CO (g) + Cl2 (g) ⇌ COCl2 (g)
A reaction mixture initially contains a CO concentration of 0.1490 M and a Cl2 concentration of 0.172 M at 1000 K.
Part A
What is the equilibrium concentration of CO at 1000 K?
Express your answer in molarity to three significant figures.
Part B
What is the equilibrium concentration of Cl2 at 1000 KK?
Express your answer in molarity to three significant figures.
Part C
What is the equilibrium concentration of COCl2 at 1000 KK?
Express your answer in molarity to three significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1
You know the right answer?
For the following reaction, Kc = 255 at 1000 K.
CO (g) + Cl2 (g) ⇌ COCl2 (g)
CO (g) + Cl2 (g) ⇌ COCl2 (g)
Questions

Computers and Technology, 20.11.2020 21:30

Mathematics, 20.11.2020 21:30



Engineering, 20.11.2020 21:30

Law, 20.11.2020 21:30

Mathematics, 20.11.2020 21:30


English, 20.11.2020 21:30

English, 20.11.2020 21:30

Mathematics, 20.11.2020 21:30

Mathematics, 20.11.2020 21:30



Mathematics, 20.11.2020 21:30


History, 20.11.2020 21:30


Engineering, 20.11.2020 21:30

History, 20.11.2020 21:30

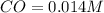
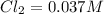

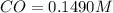
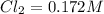
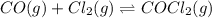
![K_c=\frac{[COCl_2]}{[Cl_2]\times [CO]}](/tpl/images/0546/7266/b0e05.png)