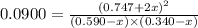Chemistry, 13.03.2020 19:56 donttrip10
A mixture of 0.590 M H 2 O , 0.340 M Cl 2 O , and 0.747 M HClO are enclosed in a vessel at 25 ° C . H 2 O ( g ) + Cl 2 O ( g ) − ⇀ ↽ − 2 HOCl ( g ) K c = 0.0900 at 25 ° C Calculate the equilibrium concentrations of each gas at 25 ° C .

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1


Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
A mixture of 0.590 M H 2 O , 0.340 M Cl 2 O , and 0.747 M HClO are enclosed in a vessel at 25 ° C ....
Questions

Mathematics, 02.08.2019 03:00

Physics, 02.08.2019 03:00

Mathematics, 02.08.2019 03:00



Mathematics, 02.08.2019 03:00

Mathematics, 02.08.2019 03:00



Social Studies, 02.08.2019 03:00



Health, 02.08.2019 03:00

Advanced Placement (AP), 02.08.2019 03:00


Mathematics, 02.08.2019 03:00

History, 02.08.2019 03:00

Mathematics, 02.08.2019 03:00


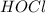 ,
, 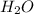 and
and 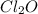 at equilibrium is, 0.215 M, 0.856 M and 0.606 M respectively.
at equilibrium is, 0.215 M, 0.856 M and 0.606 M respectively.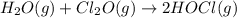
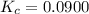
![K_c=\frac{[HOCl]^2}{[H_2O][Cl_2O]}](/tpl/images/0546/8405/da783.png)