
Chemistry, 13.03.2020 20:03 LilFreaky666
Consider these reactions, where M represents a generic metal.
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (aq) + 3 H₂ (g); ΔH₁ = − 878.0 k J
HCl (g) ⟶ HCl (aq); ΔH₂ = − 74.8 k J
H₂ (g) + Cl₂ (g) ⟶ 2 HCl (g); ΔH₃ = − 1845.0 k J
MCl₃ (s) ⟶ MCl₃ ( aq ); ΔH₄ = − 497.0 k J
Use the given information to determine the enthalpy of the reaction
2 M (s) + 3 Cl₂ (g) ⟶ 2 MCl₃ (s).

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
Consider these reactions, where M represents a generic metal.
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (...
2 M (s) + 6 HCl (aq) ⟶ 2 MCl₃ (...
Questions

Mathematics, 17.12.2020 07:30

History, 17.12.2020 07:30



Mathematics, 17.12.2020 07:30



Mathematics, 17.12.2020 07:30

History, 17.12.2020 07:30

English, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30

History, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30


Mathematics, 17.12.2020 07:30

Biology, 17.12.2020 07:30

Social Studies, 17.12.2020 07:30

Mathematics, 17.12.2020 07:30

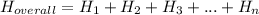 .
.

