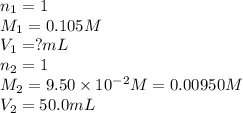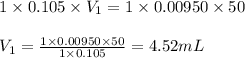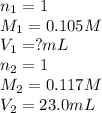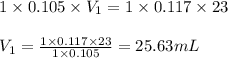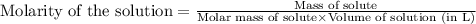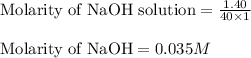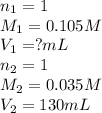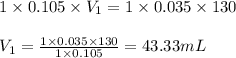Chemistry, 13.03.2020 22:21 WritingStar1313
How many milliliters of 0.105 M HCl are needed to titrate each of the following solutions to the equivalence point?
a. 50.0 mL of 9.5010?2 M NaOH
b. 23.0 mL of 0.117 M NH3
c. 130 mL of a solution that contains 1.40 g of NaOH per liter

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
How many milliliters of 0.105 M HCl are needed to titrate each of the following solutions to the equ...
Questions



Health, 05.03.2021 21:00

SAT, 05.03.2021 21:00



Chemistry, 05.03.2021 21:00


History, 05.03.2021 21:00


Chemistry, 05.03.2021 21:00



Mathematics, 05.03.2021 21:00


Mathematics, 05.03.2021 21:00


Mathematics, 05.03.2021 21:00


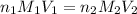
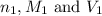 are the n-factor, molarity and volume of acid
are the n-factor, molarity and volume of acid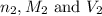 are the n-factor, molarity and volume of base
are the n-factor, molarity and volume of base