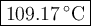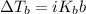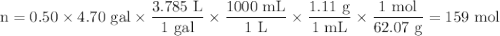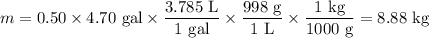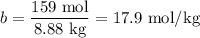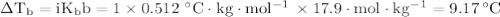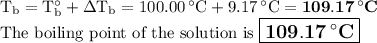Chemistry, 13.03.2020 21:58 alexchou773pejk7x
A 50/50 blend of engine coolant and water (by volume) is usually used in an automobile's engine cooling system. If a car's cooling system holds 4.70 gal, what is the boiling point of the solution? For the calculation, assume that at normal filling conditions, the densities of engine coolant and water are 1.11 g/mL and 0.998 g/mL respectively. Also, assume that the engine coolant is pure ethylene glycol ( HOCH 2 CH 2 OH ) , which is non‑ionizing and non‑volatile, and that the pressure remains constant at 1.00 atm. The boiling‑point elevation constant for water will also be needed.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:50
What is the specific heat of a substance that absorbs 2.5×10^3 joules of heat when a sample of 1.0 ×10^4g of the substance increases in temperature from 10°c to 70°c?
Answers: 2

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

You know the right answer?
A 50/50 blend of engine coolant and water (by volume) is usually used in an automobile's engine cool...
Questions


Mathematics, 18.10.2020 01:01


Arts, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01



English, 18.10.2020 01:01



Mathematics, 18.10.2020 01:01


Mathematics, 18.10.2020 01:01


Mathematics, 18.10.2020 01:01

Chemistry, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01


Biology, 18.10.2020 01:01

English, 18.10.2020 01:01