
Oxygen gas reacts with powdered aluminum according to the following reaction:
4Al(s)+3O2(g)→2Al2O3(s)
What volume of O2 gas, measured at 787 mmHg and 21 ∘C, is required to completely react with 55.0 g of Al?
Express the volume in liters to three significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Oxygen gas reacts with powdered aluminum according to the following reaction:
4Al(s)+3O2...
4Al(s)+3O2...
Questions

English, 23.06.2019 18:30

Mathematics, 23.06.2019 18:30



Chemistry, 23.06.2019 18:30



Mathematics, 23.06.2019 18:30












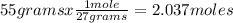
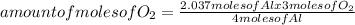
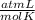 T= 21 C=294 K
T= 21 C=294 K

