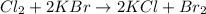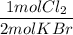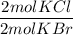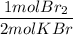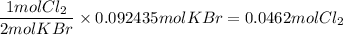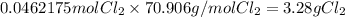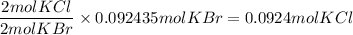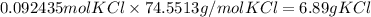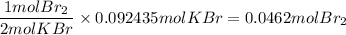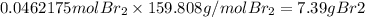What is the reaction for CL^2 + 2 KBr —> 2 KCL+Br^2 of 11 grams of potassium bromide?
Answ...

Chemistry, 13.03.2020 22:40 jacckiie5176
What is the reaction for CL^2 + 2 KBr —> 2 KCL+Br^2 of 11 grams of potassium bromide?
Answer choices:
1 mol
159.808g
7.39g
119.002g
2
2mol
11.0g
Br^2
KBr

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
Questions



Mathematics, 19.02.2021 18:20

Social Studies, 19.02.2021 18:20



History, 19.02.2021 18:20


Social Studies, 19.02.2021 18:20



Mathematics, 19.02.2021 18:20




Mathematics, 19.02.2021 18:20

Biology, 19.02.2021 18:20