
Chemistry, 13.03.2020 23:58 jwood287375
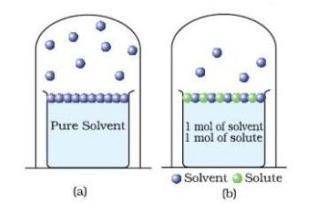
Use the model below to answer the question.
A. Student B says adding an ionic solute increases the boiling point more than adding a covalent solute.
B. Student D says adding a solute allows more water to leave (decreases the boiling point)
C. Student A says adding a solute allows less water to leave (increases the boiling point)
D. Student C says adding a solute has no effect on the boiling point

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Which organic molecule is found in the chromatin of cells?
Answers: 1

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3
You know the right answer?
Use the model below to answer the question.
A. Student B says adding an ionic solute increase...
A. Student B says adding an ionic solute increase...
Questions


Mathematics, 18.10.2020 01:01


Biology, 18.10.2020 01:01



Engineering, 18.10.2020 01:01

History, 18.10.2020 01:01

Physics, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01


Mathematics, 18.10.2020 01:01

History, 18.10.2020 01:01




Mathematics, 18.10.2020 01:01



