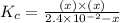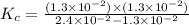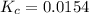Consider the following reaction:
SO2Cl2(g)⇌SO2(g)+Cl2(g)
A reaction mixture is made cont...

Chemistry, 14.03.2020 01:16 Bescobar017
Consider the following reaction:
SO2Cl2(g)⇌SO2(g)+Cl2(g)
A reaction mixture is made containing an initial [SO2Cl2] of 2.4×10−2 M . At equilibrium, [Cl2]= 1.3×10−2 M .
Calculate the value of the equilibrium constant (Kc).

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
Questions





Mathematics, 10.06.2021 01:00

Mathematics, 10.06.2021 01:00


Physics, 10.06.2021 01:00

Mathematics, 10.06.2021 01:00

Mathematics, 10.06.2021 01:00

Mathematics, 10.06.2021 01:00

English, 10.06.2021 01:00





Computers and Technology, 10.06.2021 01:00

Mathematics, 10.06.2021 01:00


Advanced Placement (AP), 10.06.2021 01:00

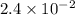 0 0
0 0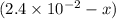 x x
x x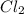 at equilibrium =
at equilibrium = 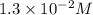
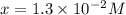
![K_c=\frac{[SO_2][Cl_2]}{[SO_2Cl_2]}](/tpl/images/0547/3049/1e2ab.png)