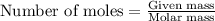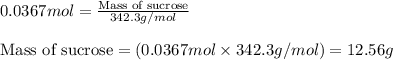Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 07:30
The compound formed from 2 atoms of hydrogen and one atom of oxygen is
Answers: 1

Chemistry, 23.06.2019 08:00
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1
You know the right answer?
A Assuming the reaction is first order in sucrose, determine the mass of sucrose that is hydrolyzed...
Questions


Mathematics, 07.10.2019 07:30

Chemistry, 07.10.2019 07:30


English, 07.10.2019 07:30


Biology, 07.10.2019 07:30


Chemistry, 07.10.2019 07:30

History, 07.10.2019 07:30

Mathematics, 07.10.2019 07:30









Geography, 07.10.2019 07:30

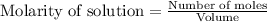
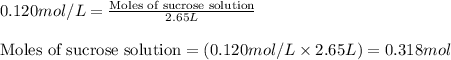
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0547/4142/f1041.png)
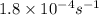
![[A_o]](/tpl/images/0547/4142/dc622.png) = initial amount of the sample = 0.318 moles
= initial amount of the sample = 0.318 moles![1.8\times 10^{-4}=\frac{2.303}{12000}\log\frac{0.318}{[A]}](/tpl/images/0547/4142/9391b.png)
![[A]=0.0367mol](/tpl/images/0547/4142/40b69.png)