

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1

You know the right answer?
2 H2S(g) ⇄ 2 H2(g) + S2(g) Kc = 9.3× 10-8 at 400ºC 0.25 moles of H2S are placed in a 3.0 L container...
Questions

Physics, 17.10.2019 23:00


Mathematics, 17.10.2019 23:00

Mathematics, 17.10.2019 23:00

English, 17.10.2019 23:00








Geography, 17.10.2019 23:00


Biology, 17.10.2019 23:00

Mathematics, 17.10.2019 23:00

Mathematics, 17.10.2019 23:00

Mathematics, 17.10.2019 23:00

English, 17.10.2019 23:00

![[H_{25}]_{initial} = \frac{0.25}{3} = 0.0833](/tpl/images/0547/6467/a1d57.png)
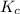 = 9.3x10⁻⁸ which is very small
= 9.3x10⁻⁸ which is very small


