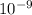Chemistry, 15.03.2020 01:53 harleycrider2251
Determine the [H3O+] in a 0.265 M HClO solution. The Ka of HClO is 2.9 × 10-8.
a. 1.3 × 10-6 M
b. 7.7 × 10-9 M
c. 1.1 × 10-10 M
d. 8.8 × 10-5 M
e. 4.9 × 10-4 M

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Determine the [H3O+] in a 0.265 M HClO solution. The Ka of HClO is 2.9 × 10-8.
a. 1.3 ×...
a. 1.3 ×...
Questions

Biology, 01.04.2021 20:30


English, 01.04.2021 20:30

History, 01.04.2021 20:30

Health, 01.04.2021 20:30


Mathematics, 01.04.2021 20:30


English, 01.04.2021 20:30

Mathematics, 01.04.2021 20:30


Chemistry, 01.04.2021 20:30

Mathematics, 01.04.2021 20:30

Mathematics, 01.04.2021 20:40


History, 01.04.2021 20:40



Mathematics, 01.04.2021 20:40

![Ka=\frac{[H_{3} O^{+}][ClO^{-}] }{[HClO]}](/tpl/images/0547/8898/f175d.png)
![2.9x10^{-8} =\frac{[x][x] }{0.265-x}](/tpl/images/0547/8898/9fe65.png)
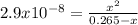
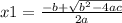 and
and 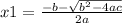
![\frac{[ClO-][H3O+]}{[HClO]}](/tpl/images/0547/8898/317ca.png)
![\frac{[x] [x]}{[0.265-x]}](/tpl/images/0547/8898/b1ba9.png)
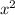 = 7.698 x
= 7.698 x