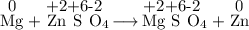Chemistry, 15.03.2020 02:21 jerseygirl6167
Consider the following reaction: Mg(s) + ZnSO4(aq) → MgSO4(aq) + Zn(s) Which of the following statements regarding this reaction is correct?
The sulfate ion is reduced.
Zinc is the reducing agent.
Magnesium is neither oxidized nor reduced.
Zinc gains two electrons.
Magnesium is the oxidizing agent.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:10
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
Consider the following reaction: Mg(s) + ZnSO4(aq) → MgSO4(aq) + Zn(s) Which of the following statem...
Questions


Chemistry, 06.10.2019 14:30

Mathematics, 06.10.2019 14:30




Mathematics, 06.10.2019 14:30

History, 06.10.2019 14:30

English, 06.10.2019 14:30


English, 06.10.2019 14:30

Geography, 06.10.2019 14:30

Mathematics, 06.10.2019 14:30






Mathematics, 06.10.2019 14:30

Biology, 06.10.2019 14:30