Chemistry, 16.03.2020 01:29 AlexRavenwood127
What volume of 0.05 mol/L HCl is required to react with 5.00g of manganese dioxide according to this equation?
4HCl(aq) + MnO2(s) → 2H2O(l) + MnCl2(aq) + Cl2(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
What volume of 0.05 mol/L HCl is required to react with 5.00g of manganese dioxide according to this...
Questions










Geography, 25.10.2019 03:43




Mathematics, 25.10.2019 03:43



History, 25.10.2019 03:43

Mathematics, 25.10.2019 03:43

Physics, 25.10.2019 03:43

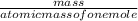
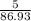
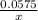 moles of MnO2
moles of MnO2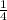 =
=