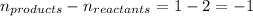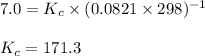Chemistry, 16.03.2020 16:11 maddie53116
The equilibrium between NO2 and N2O4 can be described by the following equation:
2NO2(g) ⇌ N2O4(g) Kp = 7.0
If a sealed flask contains 1.5 atm of NO2 and 14.2 atm of N2O4. Calculate the value of Kc for the reaction,

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
The equilibrium between NO2 and N2O4 can be described by the following equation:
2NO2(g...
2NO2(g...
Questions



English, 11.04.2021 19:20

Computers and Technology, 11.04.2021 19:20





Mathematics, 11.04.2021 19:20


Mathematics, 11.04.2021 19:20

Mathematics, 11.04.2021 19:20

World Languages, 11.04.2021 19:20

Mathematics, 11.04.2021 19:20

Business, 11.04.2021 19:20

Spanish, 11.04.2021 19:20

History, 11.04.2021 19:20

Social Studies, 11.04.2021 19:20

Mathematics, 11.04.2021 19:20

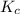 for given reaction is 171.3
for given reaction is 171.3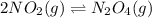
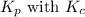 is given by the formula:
is given by the formula: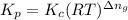
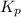 = equilibrium constant in terms of partial pressure = 7.0
= equilibrium constant in terms of partial pressure = 7.0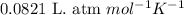
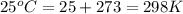
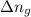 = change in number of moles of gas particles =
= change in number of moles of gas particles =