Chemistry, 16.03.2020 17:33 20emmanuelg1030
Write the precipitation reaction for calcium chloride in aqueous solution: Use the pull-down menus to specify the state of each reactant and product. CaCl2 + Is calcium chloride considered soluble or not soluble ? ... ... A. Soluble ... B. Not soluble

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1



Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Write the precipitation reaction for calcium chloride in aqueous solution: Use the pull-down menus t...
Questions

History, 21.11.2019 21:31


Mathematics, 21.11.2019 21:31


History, 21.11.2019 21:31


History, 21.11.2019 21:31


Advanced Placement (AP), 21.11.2019 21:31


Spanish, 21.11.2019 21:31

History, 21.11.2019 21:31

History, 21.11.2019 21:31






Mathematics, 21.11.2019 21:31

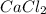 is an ionic compound as it's atoms contain a metal and a non-metal.
is an ionic compound as it's atoms contain a metal and a non-metal.